Methane (CH4) is a significant greenhouse gas with a profound impact on the environment. The global warming potential of methane is 28 times greater than that of carbon dioxide (CO2) over a 100-year period. Livestock, particularly cattle, emit significant amounts of methane (around 4.5% of total EU emissions) through enteric fermentation and manure management. The increase in methane concentrations requires urgent action to mitigate its growing environmental impact. The CANMILK project aims to develop a novel technology for methane conversion to CO2 using non-thermal plasma (NTP) and catalysts.
First results in plasma-based methane conversion
NTP produces very active species that have the ability to activate methane even at room temperature and atmospheric pressure using relatively low energy input. Initial research led by the VTT Technical Research Centre of Finland, the project coordinator of CANMILK, has produced promising results using Dielectric-Barrier-Discharge (DBD) reactors. Early laboratory results show that methane can be easily activated with NTP at as low as 23W plasma power. 54% methane conversion can be achieved at 27W, while increasing the plasma power above 30W leads to 90% methane conversion. However, as the NTP is non-selective in nature, methane is converted into both carbon monoxide (CO) and carbon dioxide (CO2). The proportion of CO₂ increased with higher plasma power but did not exceed 56% selectivity.
The Role of Catalysts
To overcome the limitations of selectivity, VTT designed and introduced various methane oxidation catalysts (MOCs). These catalysts, based on transitional metals (Copper, Iron, and Cobalt) and noble metals (Palladium and Platinum) supported on gamma-alumina (Al₂O₃), were integrated into the plasma discharge zone for best catalyst and plasma interaction.
Key observations from the catalyst enhanced system include:
- The presence of catalyst reduced the residence time of gases within the plasma discharge zone, resulting in an overall reduction in methane conversion.
- Further, the presence of catalyst inside the discharge zone increased the power input required to ignite the plasma.
- The catalyst-plasma system showed methane conversion from 27 W plasma power.
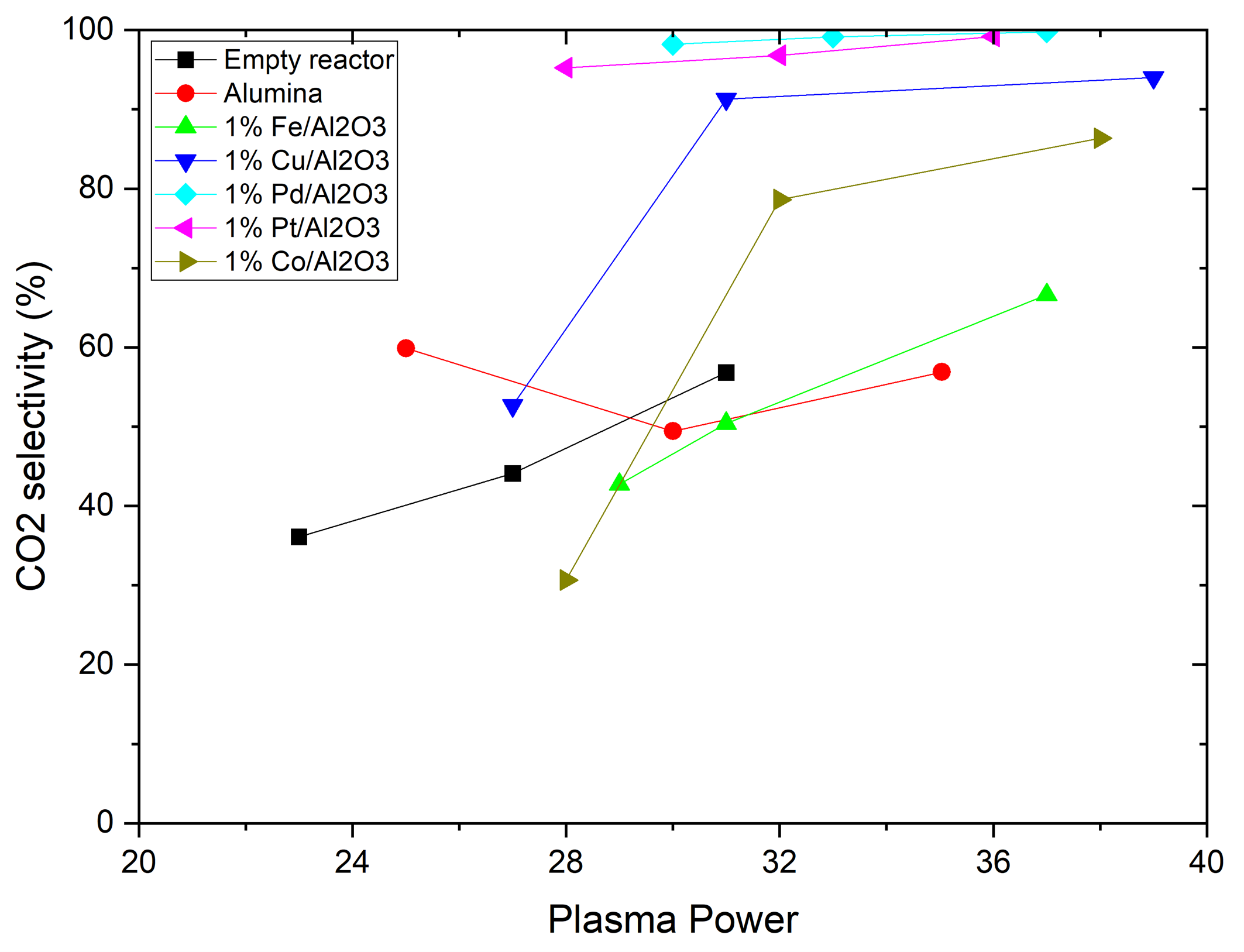
- Transition metal catalysts, particularly Cu was effective at lower plasma power levels (27 W).
- Noble metal catalysts, particularly Palladium (Pd), showed greater activity at higher plasma power, achieving 78% methane conversion at 37 W.
- Noble metal catalysts improved CO2 selectivity in comparison with transition metal catalysts and empty reactors.
- At all power levels tested, the Pd catalyst was able to achieve 99% CO2 selectivity, while the Pt catalyst achieved over 95% CO2 selectivity.
Apart from CO and CO2, there is an unwanted by product (NOx and N2O) formed during the studies. Vtt is continuously exploring different materials to minimize the formation of NOx and N2O.
Next Steps
While the early results are promising, further work is needed to evaluate the feasibility of scaling the technology for real-world applications, such as use on dairy farms. Areas for future investigation include:
- Minimising by-products: Ensuring that the process does not produce unintended emissions.
- Optimising energy use: improving the energy efficiency of the plasma catalyst system.
- Pilot Testing: Assessing performance under conditions that replicate dairy farm environments.
Potential Applications
The combination of plasma and catalyst technology represents a potential tool for addressing methane emissions in agriculture. The findings from the CANMILK project contribute to ongoing efforts to explore innovative and sustainable solutions for reducing greenhouse gas emissions. Further research and development will determine how this approach can be applied effectively at a larger scale.